Titanium does not rust. It also does not corrode in typical environments. Titanium prevails in the same condition under which iron forms rust and breaks up. Pure titanium, as well as its alloys, have excellent corrosion-resistant properties. Until you implement some particular conditions, you can trust titanium with your eyes closed. Titanium’s behaviors in natural conditions are bizarre and also inquisitive. It makes friends with oxygen and water, which are the biggest enemies of iron. The titanium also doesn’t follow the rule of the galvanic series (like a boss). There are lots of things to know about titanium so let’s go!
Does Titanium Rust?
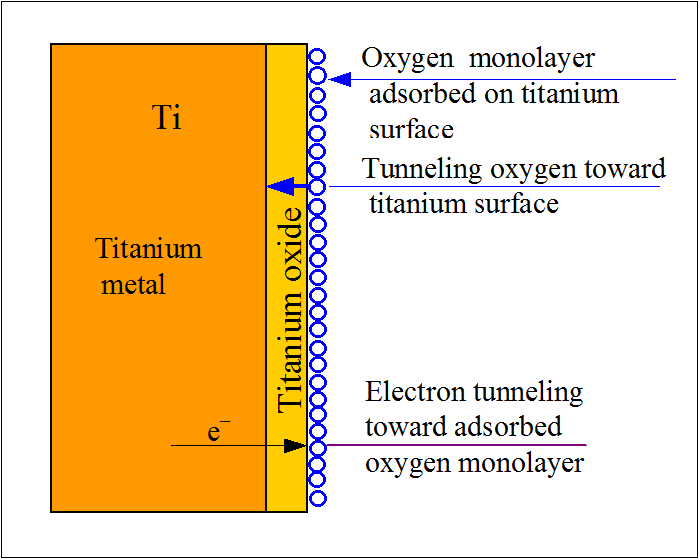
To put it simply, rust is iron oxide. It’s the reddish coating on the surface of iron that results from oxidation. The reaction is elaborate but to summarize it: when iron comes in contact with water or moisture, it reacts with oxygen and hydrogen to form hydrated iron oxide. We call it rust. Rust corrodes iron and its alloys. Other metallic elements and alloys also corrode, but not because of rust. Rust is exclusive to iron. But this term is used interchangeably with corrosion. As far as titanium is concerned, it’s a highly reactive metal. But at the same time, it is also very rust-resistant. Rust and corrosion are the results of the reactivity of a metal. So ‘rusting’ and ‘corrosion-resistant’ don’t seem to go together. That’s where titanium is exceptional. Its reactive nature protects it from corrosion. When titanium contacts with oxygen, it forms a very thin oxide layer. This layer does two things. One, it protects the underlying metal from further reacting with anything. Two, this layer steadily grows in thickness, which strengthens the protection.
Depending on the environment, the composition of the oxide can vary. In aqueous environments, titanium forms titanium dioxide (TiO2) with oxygen. In a high-temperature atmosphere, titanium oxide (TiO) is formed. However, this only applies to pure titanium. Titanium alloys are prone to rust and corrosion depending on the alloying elements. Also, the presence of oxygen is a must to protect titanium. In environments devoid of oxygen, titanium can readily corrode.
Is Titanium Rust Proof?
Titanium is rust-resistant but not completely rust and corrosion-proof. Pure titanium has the highest rust resistance property. Alloys of titanium also have similar attributes. Titanium’s susceptibility to corrosion depends on two factors mainly; the amount of oxygen or moisture and the nature of exposure. The type of alloy also plays a role. The thin layer of titanium oxide protects the metal beneath. But what if there is no oxygen? In an oxygen-free environment like a vacuum, titanium is just as vulnerable to corrosion as any other metal. Stress corrosion can occur if you expose pure titanium to methanol at <1.5% moisture. Hydrogen embrittlement is also seen in high-temperature conditions. Both decays have one characteristic in common; less than ideal oxygen concentration in the surrounding environments.
What Type of Corrosion Occurs in Titanium?
Among so many different types of corrosion, only a few are frequent in titanium and its alloys. The types of corrosion are general corrosion, crevice corrosion, hydrogen embrittlement, pitting, stress corrosion, etc.
Galvanic corrosion and corrosion fatigue usually occur in titanium. Let’s take a look at corrosion types that affect titanium:
General Corrosion
General corrosion looks like a mottled and rough metal surface. This type of corrosion takes place uniformly all over the exposed metal surface. Titanium alloys can suffer from general corrosion in tight spots on the metal surface. The tight crevices can result from poor weld joints, seal joints, etc. General corrosion may occur when the crevice is exposed to high temperature (>70oC) solutions containing chloride, fluoride, bromide, or sulfate.
Stress Corrosion
Stress corrosion occurs when a susceptible metal is subjected to constant tensile stress. Even if a metal is vulnerable to stress corrosion, it is impossible to realize it without applying optimum tensile stress. Also, stress corrosion does not equally affect all classes of titanium alloys. Specific environments like halide solutions, methanol solutions, exposure to solid or liquid cadmium, aqueous solutions of chloride, etc., paired with the right amount of stress, can inflict stress corrosion in titanium alloys.
Hydrogen Embrittlement
Hydrogen embrittlement is also known as hydrogen-induced cracking or hydrogen-assisted cracking. Titanium alloys are widely used in hydrogen-rich environments. The oxide coating protects titanium from absorbing hydrogen. The moisture in the atmosphere protects the coating. But in a dry or anhydrous condition, hydrogen can readily penetrate the layer and cause corrosion.
Why Does Galvanic Corrosion Not Occur in Titanium?
Galvanic corrosion occurs when two different metals are exposed to the same electrolyte, one metal acts as the anode and another as the cathode. Anode loses electrons to cathode. So, the metal acting as anode corrodes gradually. Which metal acts as the anode and acts as cathode depends on its place in the galvanic series. However, titanium does not reduce dissolved oxygen as efficiently as metals like copper. Due to this property, when you couple a less noble metal with titanium, the metal corrodes less. Similarly, if you couple titanium with metals higher in galvanic series, the electrode potential of titanium rises, and the corrosion rate reduces. So, it does not start to lose electrons and corrode subsequently. The reason behind this behavior is that titanium is neither an ideal anode nor a cathode in a galvanic cell. It does not accept or lose electrons unless the environment is specific, like anhydrous conditions. The oxide layer protects titanium from losing or gaining electrons. For this property, titanium is the material of choice to make dental implants.
Do Titanium Alloys Rust?
Titanium alloys are prone to similar corrosion as pure titanium. One thing to remember is the main goal of making alloys is to improve the property of a material, not to degrade it. So, all the titanium alloys have similar or improved corrosion resistance properties to pure titanium. Palladium improves titanium’s resistance to reducing acids like hydrochloric acid, sulfuric acid, etc. I talked about general corrosion above; another name for general corrosion is crevice corrosion, as it occurs in tight crevices at high temperatures. Palladium increases the critical temperature of titanium. It protects the metal from crevice corrosion in saltwater. There are also lots of alloys of titanium and they have a plethora of uses. one thing that is common among them is that they have excellent rust-resistant properties.
How to Improve the Corrosion Resistance of Titanium?
Titanium, by default, is resistant to rust and corrosion. Pure titanium does not corrode unless it’s in an oxygen-free environment. The aerospace industry and medical sector have extensive use of titanium to make various parts and products. When the environment does not take kindly to titanium or its alloys, there are alternate measures to overcome the odds.
Alloy improves the corrosion resistance of titanium. It is the most effective method to enhance the desired properties of titanium. Alloying elements are precious metals such as palladium, nickel, molybdenum, etc. A minimal amount of these metals is enough to expand the corrosion resistance of titanium by a significant margin. Besides alloying, other methods to increase the corrosion resistance of titanium are available as well like: • Increasing the thickness of the surface oxide coating. It can be achieved by thermal oxidation or anodizing. • Maintaining the surface oxide layer by anodic control or galvanic coupling with a noble metal higher than titanium. • Metal surface coating by precious metals. • Adding inhibitors to the reducing environment to further stabilize the oxide layer.
Erosion of Titanium
Erosion and corrosion are quite different. Corrosion is a chemical phenomenon, whereas erosion happens due to physical forces. Titanium is used in stressed environments like pipework systems under the sea. Turbulent waters can remove the protective oxide layer, exposing the metal surface to corroding substances.
Thanks to the regenerative ability of the oxide layer, titanium can resist such forms of erosion. Erosion is negligible for a water flow rate up to 18m/s. Titanium is also resistant to erosion by sea waters containing sand and silicon carbide granules flowing at 2m/s. If the carborundum granules are very coarse, it can cause erosion of the oxide layer and corrosion of titanium consequently. It occurs because, in such conditions, there is not enough time to regenerate the oxide layer. The erosion rate is faster than the regeneration rate. Related topics you must read Does Tungsten Rust Does Nickel Rust Does Copper Rust Does Silver Rust Does Sterling Silver Rust Does Stainless Steel Rust Is Rusting of Iron a Chemical Change Does Vinegar Remove Rust Does Brass Rust Does Platinum Tarnish
Conclusion
We often come across titanium in our everyday life considering its extensive usage in sports equipment, scissors, mobile phones, etc. But unless you did some in-depth studying and research, you are not likely to know the impressive properties of titanium. I believe this write-up could answer queries like whether does titanium rust or not and give you an overall conception of this fantastic natural element. Happy Learning!!